INTRODUCTION
Toxoplasma gondii is a protozoan parasite that can infect most animals and humans, with a worldwide distribution, permanently infecting nearly 20% of the global population [1–4]. Specific groups of patients who are immunologically impaired could be severe. Thus, prevention and diagnosis of T. gondii infection become crucial for the surveillance and control. Recent important progress has been made identifying anti-toxoplasma vaccine candidates that can stimulate an immunological response [4]. However, vaccine efficacy is not successful. The diagnosis of toxoplasmosis in humans is made by biological, serological, histological, molecular methods, parasite isolation, or by some combination of the above [5,6]. Tachyzoite lysate antigen (TLA) as a coating antigen used in conventional indirect ELISAs showed different results, resulting in difficulty to standardize and evaluation. Commercial test kits were used to determine IgM and/or IgG antibodies showed false-positive, and the reported results are difficult to interpret [6–8]. Thus, recombinant proteins as alternative approach have been used since the recombinant protein showed advantages in the precision and standardization of the antigen [6].
Numerous recombinant antigens, including granule antigens GRA1, GRA2, GRA4, GRA6, GRA7, and GRA8, rhoptry proteins ROP1 and ROP2, matrix protein MAG1, microneme proteins MIC2, MIC3, MIC4, and MIC5, and surface antigens SAG1 and SAG2, have been expressed in Escherichia coli or yeast, and their potential diagnostic value or vaccine efficacy were evaluated in humans or animals [9–14]. We recently reported that recombinant virus-like particles (VLPs), containing T. gondii inner membrane complex (IMC), have shown that VLPs are highly immunogenic [4]. Since VLPs are formed on the surface with high-density particles acting as antigens, which can induce a high immune response [15], indicating that VLPs could be used as a protein antigen for diagnostic potential or vaccine candidate.
Malaria is one of the most common infectious diseases and a great public health problem worldwide. Identification of potential diagnostic and vaccine development are particularly important. Since rhoptry protein in malaria parasite is very important in invasion of its host cells [16], and rhoptries are major players in T. gondii invasion also [17], we assume that cross-reactivity may exist between T. gondii and malaria. In this study, we, for the first time, generated virus-like particles containing T. gondii ROP4 protein. We found that T. gondii ROP4 protein can be expressed on the surface of influenza M1 VLPs. VLP protein antigens showed IgG reactivity with T. gondii-infected sera, and IgG cross-reactivity with Plasmodium berghei malaria-infected sera.
MATERIALS AND METHODS
Parasites, cells, and antibodies
Toxoplasma gondii RH strain and ME49 strain were maintained according to the methods described previously [18–20]. T. gondii RH stain was used for RNA extraction, and T. gondii ME49 was used to infect mice and to collect sera. Spodoptera frugiperda Sf9 cells were maintained in suspension in serum-free SF900 II medium (Invitrogen, Carlsbad, California, USA) at 27°C in spinner flasks at 130 to 140 rpm. S. frugiperda Sf9 cells were used for production of recommended baculovirus (rBV) and virus-like particles. Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) was purchased from Southern Biotech (Birmingham, Alabama, USA).
Cloning of T. gondii rhoptry protein (ROP4) and influenza M1
T. gondii RH strain was collected from mice, and RNA was extracted using RNeasy Mini Kit (Qiagen, Valencia, California, USA). Total RNA was reversely transcribed to cDNA using Prime Script 1st strand cDNA synthesis kit according to the manufacturer’s instructions (Takara, Otsu, Japan). T. gondii ROP4 gene was amplified by PCR from cDNA with primers. The primers were designed according to the nucleotide sequence of ROP4 in GenBank (accession no. EU047558): forward (5′-AAAGCATGCACCATGGGGCACCCTACCTCTTT-3′) and reverse (5′-TTAGGTACCTCACGTTTCCGGTGGTGGCAT-3′) with SphI and KpnI restriction enzyme sites (underlined). PCR product was cloned into pFastBac vector (Invitrogen) as described previously [21]. For influenza M1 (accession no. EF4 67824, 1,027 bp) gene cloning, A/PR/8/34 virus was inoculated into MDCK cells, and total viral RNA was extracted as mentioned above. Reverse transcription (RT) and PCR were performed on extracted viral RNA using the 1-step RT-PCR system (Invitrogen) with gene specific oligonucleotide primers. The following primer pairs were used for M1: 5′-TCCCCCGGG CCACCATGAGCCTTCTGACCGAGGTC-3′; reverse primer, 5′-TTACTTCTAGATTACTTGAACCGTTGCATCTG-3′; SmaI and XbaI sites are underlined. A cDNA fragment containing the M1 was cloned into pFastBac vector (Invitrogen). The recombinant plasmids ROP4-pFastBac or M1-pFastBac were transformed into an E. coli DH5-α. The targeted fragments of the ROP4 gene and M1 gene were identified by restriction digestion and sequencing analysis. Confirmed recombinant plasmids were transformed into a DH10-Bac and extracted using FavorPrep gel purification Kit (Favorgen, Cheshire, UK). The recombinant plasmid DNAs (DH10-Bac) were stored at −20°C until used.
Generation of recombinant baculovirus (rBV) and VLPs
To generate rBV, transfections of recombinant plasmid ROP4-pFastBac or M1-pFastBac were transfected into the Sf9 cells using cellfectin II (Invitrogen) as according to the manufacturer. To produce VLPs containing ROP4 and M1, Sf9 cells were coinfected with rBVs expressing ROP4 or M1. VLPs released into the cell culture supernatants were harvested 3 days after infection and cleared by centrifugation at 6,000 rpm for 30 min at 4°C to remove cells. Supernatants containing VLPs were concentrated by high-speed centrifugation (45,000 g for 30 min) and purified through a 15–30–60% discontinuous sucrose gradient at 45,000 g for 1 hr at 4°C. VLP bands between 30% and 60% were harvested and pelleted by high-speed centrifugation (45,000 g for 30 min). The VLPs were resuspended in 0.1 M PBS overnight at 4°C and concentration was determined using QuantiPro BCA Assay Kit (Sigma-Aldrich, St. Louis, Missouri, USA).
Characterization of VLPs
To characterize the VLPs, the morphology was confirmed by electron microscopy. For electron microscopy, negative staining of VLPs was performed followed by transmission electron microscopy as described previously (Tecnai G2 spirit, FEI, Hillsboro, Oregon, USA) [21].
Reactivity of VLPs with T. gondii-infected mouse sera
Female inbred BABL/c (aged 8 weeks; Nara Biotech, Kyonggi-do, Korea) were used. All animal experiments and husbandry involved in these studies were conducted under the guidelines of the Kyung Hee University IACUC. Mice were infected with T. gondii (ME49), and the mouse sera were collected at week 4 and stored at −20°C until used. VLPs containing T. gondii ROP4 protein was identified by western blot. Monoclonal mouse anti-M1 antibody was used to confirm influenza M1 protein content. The levels of IgG antibody was determined by ELISA. Flat-immunoplates (96-well) were coated with 100 μl of VLPs at a concentration of 4 μg/ml in 0.05 M carbonate bicarbonate buffer (pH 9.6) per well and incubated overnight at 4°C. The mouse serum samples were serially diluted in PBST (100 μl/well) and incubated in the plates for 1.5 hr at 37°C. Horseradish peroxidase (HRP)-conjugated goat anti-mouse immunoglobulin G (IgG) was purchased from Southern Biotech.
IgG cross-activity of VLPs with Plasmodium berghei antibody
IgG cross-reactivity of VLPs was determined by reacting with Plasmodium berghei-infected mouse sera. P. berghei was kindly provided by Dr. YC Hong at Kyungpook National University. Mice were infected with P. berghei, and mouse sera were collected at 4 weeks and stored at −20°C until used. ELISA was performed to determine IgG cross-reactivity between T. gondii and P. berghei. T. gondii-, and P. berghei-infected mouse sera were used for primary antibodies at 1:50 dilution, and HRP-conjugated goat anti-mouse IgG antibodies were used as secondary antibodies.
RESULTS
Recombinant constructs generated
T. gondii ROP4 and influenza M1 genes were amplified by PCR or RT-PCR, respectively. As shown in Fig. 1, ROP4 was 1,728 bp (A), and M1 was 1,027 bp (B) in size, respectively. The amplified PCR products were cloned into pFastBac vectors, and the insertions of ROP4 and M1 in pFastBac vectors were confirmed by restriction enzymes (Fig. 2A, B). The nucleotide sequences of the T. gondii ROP4 and influenza M1 genes were identical to previously published sequences (accession nos. EU047558 for T. gondii ROP4 and EF467824 for M1) by DNA sequencing (Eurofins MWG Operon).
Influenza M1 VLPs produced
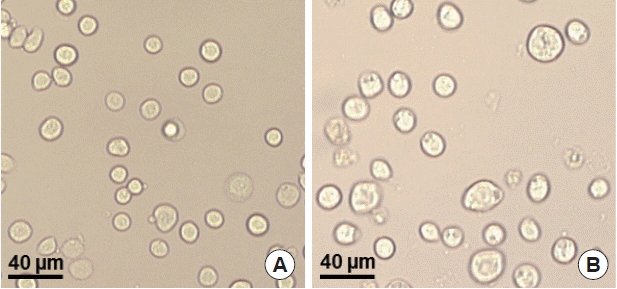
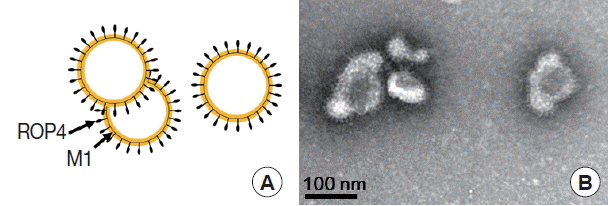
To produce M1 VLPs, recombinant baculovirus (rBV) expressing T. gondii ROP4 or influenza M1 were co-infected into Sf9 cells, and thus, M1 VLPs were produced. The VLP-producing Sf9 cells (Fig. 3B) are significantly larger in size than normal control Sf9 cells (Fig. 3A). The size and morphology of influenza VLPs were examined under electron microscopy. M1 VLPs showed spherical shapes with spikes on their surfaces, and generated M1 VLPs resembled virions in morphology and size (Fig. 4A, B).
Influenza M1 VLPs reacted with sera from T. gondii (ME49)-infected mice
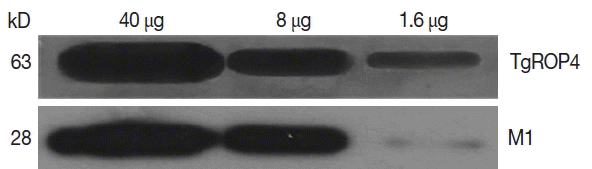
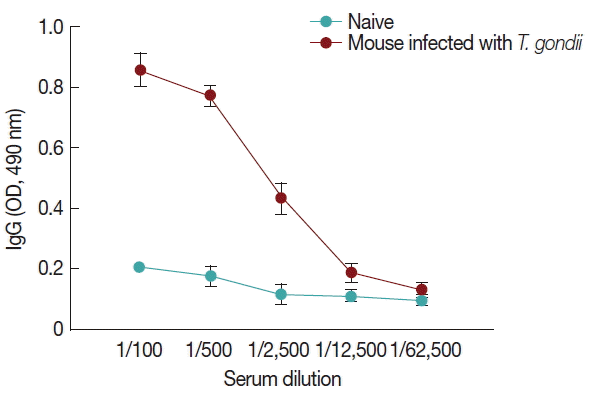
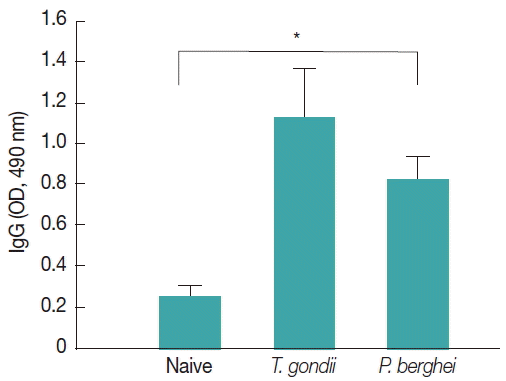
Influenza M1 VLPs containing ROP4 were used to react with antibodies from T. gondii-infected mice by western and ELISA. As seen in Fig. 5, the incorporation of T. gondii ROP4 (63 kDa) and influenza M1 (28 kDa) into VLPs was confirmed by western blot. As seen in Fig. 6, compared to naïve control mouse sera, high levels of IgG antibodies were detected from T. gondii-infected mouse sera at 1:100, 1:500, and 1:2,500 dilutions when M1 VLPs were used as coating antigens by ELISA.
Influenza M1 VLPs induced IgG cross-reactivity with Plasmodium berghei-infected sera
Influenza M1 VLPs containing T. gondii ROP4 were used to determine IgG cross-reactivity with antibodies from P. berghei-infected mice. As seen in Fig. 7, compared to naïve mouse control, higher levels of IgG antibodies were detected from P. berghei-infected mouse sera, indicating IgG cross-reactivity between T. gondii and P. berghei.
DISCUSSION
In this study, for the first time, we successfully cloned T. gondii ROP4 gene into pFastBac vector and generated baculovirus expressing T. gondii ROP4. Influenza M1 protein expressed by baculovirus expression system contributed to the formation of influenza-like shape of VLPs [22]. During the VLP generation, baculoviruses expressing T. gondii ROP4 or M1 incorporated into VLPs, which showed spherical shapes (M1) with spikes (T. gondii ROP4) on the VLP surface [4]. We found that VLP proteins were clearly recognized by polyclonal antibodies of T. gondii by western blot (Fig. 5). VLPs reacted with antibodies from mouse infected with T. gondii (Fig. 6), indicating VLPs consisting of T. gondii ROP4, could be used as a coating antigen for diagnostic and/or vaccine candidate against T. gondii infection. To determine the cross-reactivity between T. gondii and malaria, in our current study, malaria mouse model P. berghei was used. VLPs containing T. gondii rhoptry structure showed IgG cross-reactivity with P. berghei-infected mouse sera (Fig. 7), indicating that VLPs could be used as a vaccine candidate against P. berghei infection as well. Further studies are needed to elucidate their potential as a vaccine candidate against T. gondii and other coccidian species infections having rhoptry structures.
Although, a number of different recombinant proteins of T. gondii expressed by E. coli have been used as antigens for diagnostic tool or vaccine candidates, the vaccine efficacy induced by the recombinant proteins expressed by E. coli is largely limited. Mice immunized with the recombinant protein vaccines mostly showed little protection against challenge infection [23–26]. In our recent study, we have reported that VLPs targeted T. gondii inner membrane complex sub-compartment (IMC) induced humoral and cellular immunity, resulting in protection (100% survival) [4]. This promising result indicated that VLPs could be an alternative strategy for the novel vaccine [4]. The VLPs contained repetitive high density displays of T. gondii IMC surface proteins induced systemic and mucosal immune responses [15].
ROP4, a member of the prominent ROP2-protein family, is released from rhoptries, participating in the parasite-host cell penetration process [27]. Additionally, ROP4 antigen expressed by E. coli elicits strong cellular and humoral immune responses in immunized mice, which are partly protective against T. gondii challenge [28]. This T. gondii ROP4 protein is also useful as a diagnostic tool for a serological test [29]. Thus, in this study, we, for the first time, successfully produced VLPs containing T. gondii ROP4 protein in which influenza matrix M1 is as a core protein. For further studies, the protective immunity induced by VLPs immunization is needed as well as the evaluation of VLPs as a diagnostic tool for a serological test.