AbstractBovine trypanosomiasis is a significant health concern for livestock intensification in Côte d’Ivoire. This study aimed to determine the prevalence and distribution of pathogenic trypanosomes and identify the most infected cattle breed in northern Côte d’Ivoire. We examined 700 cattle and found that polymerase chain reaction (PCR) was more sensitive (12.3%) than microscopic observation (5.6%). Among the trypanosome species detected in naturally infected cattle, Trypanosoma vivax was 7.3%, Trypanosoma simiae tsavo was 6.7%, and Trypanosoma congolense was 0.4%. The overall prevalence of trypanosome infection in all cattle breeds was 12.3%, while the prevalence in individual breeds was 14.8%, 7.3%, 10.6%, and 12.3% for N’Dama, Baoule, Zebu, and Mere breed, respectively. The infected animals had low packed cell volume, influencing the prevalence. Our findings indicate that bovine trypanosomes are prevalent in Côte d’Ivoire, and their prevalence varies by region and breed. These pathogens include T. vivax, T. simiae tsavo, and T. congolense.
IntroductionTrypanosomiasis is a significant constraint to livestock development in sub-Saharan Africa [1]. Organisms belonging to the genus Trypanosoma, which are transmitted mainly by the tsetse fly, cause this disease. Tsetse flies have an area of distribution of over 10 million square kilometers in Africa, representing 38 countries [2]. The tsetse flies mainly transfer the trypanosomes from one animal to another. Trypanosome infections have a significant socio-economic impact and limit animal productivity in all African countries, including Côte d’Ivoire [3]. Nevertheless, its epidemiology is still unclear, unlike Human African Trypanosomiasis, whose epidemiology has been elucidated through advanced research. Although Côte d’Ivoire has mostly eliminated Human African Trypanosomiasis as a public health problem [4,5], evolving toward zero transmission, the epidemiology of animal trypanosomiasis is unclear in this country. The productivity losses due to African Animal Trypanosomiasis are approximately 30% [6]. Surveys conducted by Camus in 1979, Bokar in 1997, Acapovi in 2009, and Yao in 2015 have shown that the disease is most prevalent in the livestock areas of northern Côte d’Ivoire, particularly, in the departments of Korhogo, Ferkessédougou, and Boundiali, with a prevalence rate ranging between 11% and 21% [7]. Despite the control efforts made by the state of Côte d’Ivoire, animal trypanosomiasis remains uncontrolled, especially in bovines. Some trypanosome species that are pathogenic to humans have been identified in animals in the department of Sinfra [8,9]. The vulnerability of humans and their livestock to trypanosomiasis is amplified by the insufficient information regarding the status of trypanosomiasis and inadequate vector control regimes adapted to risk areas and sustainable means of control. Therefore, understanding the status of infection is essential for the appropriate control of African trypanosomiasis.
This study aimed to understand the epidemiology of bovine trypanosomiasis, understand the distribution of trypanosome species, and identify the most infected livestock breed in Côte d’Ivoire.
Materials and MethodsEthics statementThe samples were collected within the framework of epidemiological surveillance activities and supervised by the National Laboratory for Support to Agricultural Development (LANADA) after approval from the Ministry of Animal Resources Halieutics (MIRAH). Local authorities do not require an ethical statement for epidemiological surveillance efforts. Any veterinarian can draw blood from domestic animals as part of a preventative or diagnostic campaign with the farmer’s consent. No samples were collected outside those required for standard screening and diagnostic procedures. After explaining and clarifying the study’s goals, the farmers agreed to provide the blood samples from their animals, all of whom received free deworming medication. The wounded animals were cleaned and disinfected.
Study areaThis study was conducted in the ‘Savannah’ region of northern Côte d’Ivoire, bordered by Mali and Burkina Faso, from August to November 2021 [10]. It has an area of 40,323 km2 and a population of 1,388,142 inhabitants as estimated in 2012. Its climate is dry tropical, also called the Sudanese climate, which is marked by a wet season characterized by a water surplus lasting 4 to 5 months (June to September or October), alternating with a dry season lasting 7 to 8 months [11]. The dry season is marked by no rainfall, persistent cold and dry winds often laden with fine dust, known as the Harmattan, which blows from December to February. The vegetation consists of savannahs and dry forests, with residual patches representing the sacred woods of the Senoufo ethnic group. The gallery forests that border certain waterways complete these rare dense forests [12]. Overall, the vegetation of a mixture of open forest and Savannah, belonging to the sub-Sudanese domain characterized by vegetation with both herbaceous and woody strata.
Study design, Sample Estimation, and Farm Selection CriteriaWe performed cross-sectional research to assess the prevalence of trypanosomiasis in cattle by randomly selecting animals from each location. The total number of cattle was determined based on the prevalence reported in previous studies. The formula N=P (1−P) *z2/i2 described by Thrusfield [13], was used with a precision of 5% and a confidence interval (CI) (standard value 1.96) to determine the sample cattle number. As shown previously, the prevalence of bovine trypanosomiasis in Côte d’Ivoire ranged between 11% and 21%. Using this formula, the number of sample cattle was estimated to be between 601 and 1,213. In practice, depending on the size of the department, sampling was conducted in 3 to 8 villages, which were selected according to the following criteria: i) the distance between the villages should be such as to cover the department and consider the cardinal points, ii) the presence of watercourses and gallery forests near the village or the cattle yard, iii) the presence of cattle in the village, iv) the accessibility to the farm, and v) the agreement of the populations to participate in the study. N: sample size (601<N<1,213); P: expected disease prevalence (11%<P <21%); Z: 1.96 constant, i: half of the CI desired 0.05 (I=0.025).
Blood sample collectionBefore each collection, the vital signs of each animal were visually examined, such as mucosal condition, respiration, heart rate, pulse, body temperature, lymph nodes, and animal behavior (standing posture). Animals with poor vital signs were given a preference for blood sampling. After appropriate immobilization, we sampled 700 cattle twice each. The first sample was taken from the jugular vein using EDTA tubes, which were kept in cold storage until further molecular analysis. The second sampling was performed with 2 heparinized capillary tubes from the marginal ear vein after disinfecting it with 70% alcohol. One was used for microscopic examination, and the second was sealed with wax at one end to examine the PCV. In addition to the blood samples, farm and individual cattle data, such as age, breed according to the breeder, sex, and body condition score were recorded on a sample card for each herd.
Parasitological examinationAll parasitological diagnostic tests and procedures were performed as previously described [11]. Two microhematocrit capillary tubes with sealed outer ends were filled up to 3/4th with blood. One of these tubes was centrifuged at 12,000 rpm for 5 min using a hematocrit centrifuge. After centrifugation, the blood volume was measured using a hematocrit reader to assess the degree of anemia. The PCV was determined and expressed as a percentage of the total blood volume, taking values of total blood PCV [14].
The second capillary tube was used for thin blood smears. The slides were then quickly air-dried and protected from dust and insects. The smears were fixed by immersing them in methanol for 5 min and stained using a 1:10 solution of 0.4% Giemsa and distilled water for 20 min. After staining, the slides were rinsed thoroughly with tap water and dried before observation.
The trypanosomes were detected based on their morphology by observing the blood smears using a microscope (Novex Holland-B-Series) at 100×magnification. For positive cases, the morphology of the species was distinguished based on their appearance, shape, location, kinetoplast size, position and attachment of the nucleus, and flagellar length. However, this requires an experienced technician. The polymerase chain reaction (PCR) method was performed to better identify trypanosome species.
DNA extraction and PCR amplificationDNA from the blood samples was extracted using a commercially available kit (Quick-DNA Miniprep Plus Kit; Zymo Research, Irvine, CA, USA) according to the manufacturer’s instructions. The trypanosomes were identified by ITS1 PCR previously described [15]. The reaction mixture consisted of 2 μl of template, 1X of OneTaq buffer, 200 μM of each dNT, 1 μM of each primer, and 0.5 U of OneTaq DNA polymerase (NEW ENGLAND Biolabs, Ipswich, MA, USA), adjusted at 25 μl with nuclease-free water. The cycling conditions were 94°C for 5 min; 35 cycles: 94°C for 40 sec, 58°C for 40 sec, 72°C for 90 sec; 72°C for 5 min.
We also performed nested ITS PCR described by Cox A [16] targeting the partial 18S, ITS1, 5.8S, ITS2, and partial 28S regions using 25-μl reaction mixtures. The first run reaction mixture consisted of 1X of One Taq buffer, each dNTP at 200 μM, each primer (ITS1 and ITS2) at 0.4 μM, 1.25 U of One Taq DNA polymerase (NEW ENGLAND Biolabs), and 1 μl of DNA. For the second run, the first PCR product (2 μl) was added in 23 μl of a fresh mix prepared using ITS3 and ITS4 primers. The cycling conditions of both runs were 95°C for 5 min; 35 cycles: 94°C for 1 min, 55°C for 1 min, 72°C for 120 sec; 72°C for 5 min.
To confirm the different trypanosome species, 7 species-specific primers were used. PCR was performed in a 25-μl reaction mixture with 1X of One Taq buffer, each dNTP at 200 μM, each primer at 1 μM, and 0.5 U of One Taq DNA polymerase (NEW ENGLAND Biolabs). The cycling conditions were 95°C for 3 min; 40 cycles: 95°C for 30 sec, 55°C for 30 sec, 72°C for 1 min; and 72°C for 5 min. The PCR products were electrophoresed using 1.5% agarose gels (BioTools Inc, Gunma, Japan) in (TBE) buffer and stained using Safe View (4 μl for 100 ml of TBE) before being visualized under UV light. The expected fragment sizes for individual trypanosome species are shown in Supplementary Table S1.
Statistical analysisThe statistical analyses were done using the R 4.2.0 software. Arcgis 9.3 and Qgis 4.6 were used to generate the maps. The Chi2 test highlights the relationships between the parasitological and molecular results with the risk factors. The variables associated with the prevalence were determined by a mixed logistic regression using as a response variable the infection event defined by a Bernoulli random variable (0=not infected and 1=infected). The animal variable was considered as the randomization variable, and the PCV, breed, region, department, age, and sex were considered as explanatory variables. We created the following starting model:
p: is the probability that the animal is infected; Ani is an animal (i=1,...,700); Pj the value of the PCV (j=1,…..700); Rak the race of the animal (k=1,…4); Rel the region (l=1,…3); Dem department (m=1,….10); Agn the age of the animal (n=1,…4); Seo the sex of the animal (o=1,2) et £ijklmno Is the residual that follows the normal distribution N(0,σ2).
Using the likelihood method, the model was fitted with a log-likelihood approximation using the likelihood ratio test to study the influence of the explanatory variables. The same model was used to study the molecular and parasitological prevalence. Depending on all the explanatory variables, several models were tested. The best models were selected according to the Akaike information criterion (AIC). Thus, for the parasitology and molecular methods, we selected the following models, respectively:
The mean hematocrit levels were compared by ANOVA test according to infestations with different trypanosome species.
ResultsIn total, 700 animals were sampled in 79 farms in 74 villages distributed in the 10 departments of the 3 regions of the Savannah district (Supplementary Table S2).
Trypanosomes species, Abundance, and Geographical distributionWe identified 3 species of trypanosomes using classical and nested PCR, including T. vivax, T. congolense, and T. simiae tsavo (Fig. 1). T. vivax (7.28%) was the most abundant, followed by T. simiae tsavo (6.71%) and finally, T. congolense (0.42%). T. congolense was observed only in 2 departments, namely, M’bengue and Kouto. T. vivax and T. simiae tsavo were found in all departments of the Savannah district, as shown in the map (Fig. 2). To our knowledge, this is the first report of T. simiae tsavo in Côte d’Ivoire.
Comparison between Microscopy and PCR results
Table 1 summarizes the comparison of microscopy and PCR diagnostic results. The chi-square test applied to the data showed a significant difference (P<0.001) between the prevalence rates obtained using microscopy and PCR.
The prevalence obtained using microscopy was 5.6% among the 700 individuals sampled. The prevalence between different regions was also statistically different, with a slight preponderance in the Poro region. The prevalence according to the modalities of the other factors was not statistically different. The prevalence obtained by PCR was 12.3%. Except for race, all other factors, such as region, department, age, and sex, were statistically different (P<0.01) (Table 1).
Factors influencing the prevalenceAmong the models used for microscopy-diagnosed infections, the model with PCV, region, and age as fixed effect variables (AIC=262.18) was the best compared with the one with PCV, race, region, department, age, and sex (AIC=266.63). Thus, this model was selected. However, among these 3 variables, only the PCV (P<0.01) had a significant association adjusted on the other variable (region and age) with the infection.
For molecular diagnostics, the model with packed cell volume (PCV), race, region, age, and sex (AIC=466.98) was the best model than that one with PCV, race, region, department, age, and sex (AIC=468.94). According to this model, it is only the region (P<0.001) that showed a highly significant association between animal infection with race, region, age, and sex (Table 2).
Relation between packed cell volume, prevalence, and trypanosome speciesThe overall average of PCV was 35.3%±12.53%. The minimum and maximum observed were 10% and 90%, respectively. A significant difference (P<0.01) was observed between the PCV classes (Table 3). Animals with a PCV<25 were more prevalent than those with a PCV>25.
Depending on the species of trypanosome responsible for the infection (Table 4), a significant difference (P<0.05) was observed between the mean PCV of the animals. The mean PCV of the cattle infected with T. congolense (28±6.55) was significantly lower (P<0.05) than that of the animals infected with T. vivax (35.83±12.53) and T. simiae tsavo (36.16±9.83).
DiscussionOur results show that T. vivax was the predominant species responsible for bovine trypanosomiasis in the Savannah district, consistent with previous reports [1,2]. The predominance of T. vivax could be explained by the agroclimatic conditions that favor the development of some riverine tsetse flies (G. tachinoides and G. palpalis gambiensis) and Savannah-type tsetse flies (G. morsitans and submorsitans), which are all known to be potential vectors of trypanosomes, particularly T. vivax [17]. Also, tabanids are found in our study area (Savannah district), which are known mechanical vectors for T. vivax [18].
Trypanosoma simiae tsavo was the second most abundant species in our study area, which can be attributed to the laboratory techniques. Microscopic examination of parasites has limited sensitivity and specificity [19] and requires highly skilled technicians to identify trypanosomes. In case of PCR, very few studies have used this technique due to its high cost and limited accessibility. Moreover, in all the studies conducted in Côte d’Ivoire using molecular tools [20], only specific primers (microsatellites) were used to diagnose trypanosomes, considering only the 3 species: T. congolense, T. vivax, and T. brucei. Therefore, it is possible that the T. simiae tsavo species exists but could not be detected due to the differences in previously used protocols and diagnostic methods, especially the non-use of multi-species primers.
In contrast to the other species, we observed a low abundance of T. congolense, detected in only 2 departments of our study area. This prevalence could be explained by the recent reduction in the population of G. morsitans submorsitans in West African areas, including Côte d’Ivoire [21]. Indeed, previous studies [22] proved the correlation between the presence of G. morsitans submorsitans and the transmission of T. congolense. The lack of contact between cattle and G. morsitans submorsitans could explain the difference in the prevalence between T. congolense and other encountered trypanosome species.
The absence of T. brucei could be explained by the conditions that favor trypanosomes with a short development cycle. This is because the prevention of infections has been increased due to the routine use of trypanocides by farmers, which does not favor T. brucei with a long development cycle. According to [23], trypanocides represent 44% of the total veterinary drug market in sub-Saharan Africa. Moreover, antiparasitic drugs, including trypanocides, are the most sold veterinary drugs in Côte d’Ivoire [24].
The PCR diagnostic technique was more sensitive and specific than the microscopy, consistent with several previous reports [3]. Although relatively sensitive, it is not possible to detect all the positive cases using microscopy [19].
The infection was observed in all age groups except animals older than 9. The infection rate in young animals under 2 years of age was low compared with the other age groups. Similar observations have been reported by [25] in the department of Korhogo in Côte d’Ivoire. Indeed, young animals are more closely monitored for health problems. Moreover, as they are kept on the picket all day while the rest of the herd are allowed to graze, they are less in contact with the vectors of bovine trypanosomiasis. This low frequency of contact between the young animals and the vectors is advantageous for them due to less exposure to the vectors that transmit trypanosomes.
The N’Dama breed had the highest prevalence, followed by the Méré, Zebu, and Baoule breeds in decreasing order of prevalence. The local N’Dama and Baoule breeds are taurines that represent the Ivorian genetic heritage [26] and are known for their trypanotolerance. However, our results revealed that the N’Dama breed is susceptible to infections. Notably, this breed has strong genetic pressure due to uncontrolled crossbreeding by breeders. Resultantly, the breeders can no longer distinguish the population of these crosses. Hence, the products of the crosses between zebu and taurine (N’Dama, Baoule, and lagunaire cattle) are grouped under the name of Méré. This new breed is stabilizing and accounts for 80% of the cattle population in Côte d’Ivoire [27]. Notably, all projects aimed at improving cattle production in Côte d’Ivoire have used the N’Dama as a reference breed [28], making it difficult to obtain purebred trypanotolerant taurines.
Animals with trypanosome infections generally suffer from anemia. The low PCV observed in this study has also been reported previously [29]. Furthermore, using the model, this study shows that the infections diagnosed using the microscopy method and PCV are related. Indeed, the anemia or PCV percentage indicates good production performance of an infected animal. Additionally, we found that the average PCV of animals infected with T. congolense is low compared to that of animals infected with other trypanosome species, consistent with previous studies [30] that showed similar pathogenicity among T. congolense species.
Our findings indicate that bovine trypanosomiasis is prevalent in Côte d’Ivoire, varying from region to region. These pathogens include T. vivax, T. simiae tsavo, and T. congolense. Unlike other species, T. congolense was observed only in 2 departments, M’bengue and Kouto. PCR was more specific and sensitive than microscopy in diagnosing infections. The most infected breed was the N’Dama breed. PCV is a key indicator for the rapid diagnosis of trypanosomes.
AcknowledgmentsThis study was funded by the Partnership for Skills in Applied Sciences, Engineering and Technology (PASET) through the Regional Scholarship and Innovation Fund (RSIF) awarded to Jean-Yves EKRA to carry out doctoral studies at SACIDS Africa Centre of Excellence for Infectious Diseases, SACIDS Foundation for One Health, Sokoine University of Agriculture, Morogoro, Tanzania. The funders had no role in study design, data collection and analysis, the decision to publish, or the preparation of the manuscript. The findings and conclusions of this study are those of the authors and do not necessarily represent the views of the funders.
We acknowledge the Ministry of Animal Resources Halieutics (MIRAH) of Côte d’ivoire for facilitating the collection of field data. We acknowledge the Professional Organization of breeders of Ferkessedougou (OPEF) and the agents of the MIRAH for guiding us in the field. We acknowledge the breeders who participated in this study.
We acknowledge the institute Pierre Richet (IPR) of Bouake (Côte d’Ivoire), where the molecular work was conducted. We acknowledge the assistance of the staff at SUA and Southern Africa Centre of Excellence for Infectious Diseases, the SACIDS Foundation for One Health, SUA, Morogoro, Tanzania.
NotesAuthor contributions
Conceptualization: Ekra JY
Data curation: Ekra JY
Formal analysis: Ekra JY
Methodology: Ekra JY, N’Goran EK, Mafie EM
Project administration: Mafie EM
Software: Ekra JY
Supervision: N’Goran EK, Mboera LEG, Gragnon BG, Mafie EM
Validation: Mafie EM
Visualization: Mboera LEG, Assovié KRN
References1. Yao LS, Gragnon BG, Dri KLN, Coulibaly B, Komono D. Prévalence trypanosomienne dans le bassin cotonnier en zone soudanaise de Côte d’Ivoire. Rev Mar Sci Agron Vét 2020;8:417-424 (in French).
2. Acapovi-Yao G, Desquesnes M, Hamadou S, N’Goran E. Prévalence parasitologique et sérologique des trypanosomoses chez trois races bovines en zones à glossines et présumée indemne, Côte d’Ivoire. Agron Africaine 2010;21(2):205-213 (in French).
3. Kizza D, Ocaido M, Mugisha A, Azuba R, Nalule S, et al. Prevalence and risk factors for trypanosome infection in cattle from communities surrounding the Murchison Falls National Park, Uganda. Parasit Vectors 2021;14(1):1-7 https://doi.org/10.1186/s13071-021-04987-w
4. Koné M, Kaba D, Kaboré J, Thomas LF, Falzon LC, et al. Passive surveillance of human african trypanosomiasis in côte d’ivoire: Understanding prevalence, clinical symptoms and signs, and diagnostic test characteristics. PLoS Negl Trop Dis 2021;15(8):1-19 https://doi.org/10.1371/journal.pntd.0009656
5. Franco R, Cecchi G, Priotto G, Paone M, Diarra A, et al. Monitoring the elimination of human African trypanosomiasis at continental and country level: Update to 2018. PLoS Negl Trop Dis 2020;21. 14(5):e0008261 https://doi.org/10.1371/journal.pntd.0008261
6. Douati A, Kupper W, Kotia K, Badou K. Contrôle des glossines (Glassina: Diptera, Muscidae) a l’aide d’écrans et de pièges (méthodes statiques): bilan de deux années de lutte à Sikasso dans nord de la Côte- d’Ivoire. Rev Elev Med Vet Pays Trop 1986;39(2):213-219 (in French).
7. Acapovi-Yao G, Cisse B, Koumba CRZ, Mavoungou JF. Infections trypanosomiennes chez les bovins dans des élevages de différents départements en Côte d’Ivoire. Rev Med Vet (Toulouse) 2016;167(9–10):289-295 (in French).
8. Jamonneau V, Barnabé C, Koffi M, Sané B, Cuny G, Solano P. Identification of Trypanosoma brucei circulating in a sleeping sickness focus in Côte d’Ivoire: assessment of genotype selection by the isolation method. Infect Genet Evol 2003;3(2):143-149 https://doi.org/10.1016/s1567-1348(03)00069-8
9. Traoré BM, Koffi M, N’djetchi MK, Kaba D, Kaboré J, et al. Free-ranging pigs identified as a multi-reservoir of Trypanosoma brucei and Trypanosoma congolense in the Vavoua area, a historical sleeping sickness focus of Côte d’Ivoire. PLoS Negl Trop Dis 2021;15(12):1-19 https://doi.org/10.1371/journal.pntd.0010036
10. Aubertin C. Histoire et création d’une région “sous-développée” (le Nord ivoirien). Cah ORSTOM 1983;19(1):23-57.
11. Avenard JM. Le milieu naturel de la Côte-d’Ivoire. Etud Rurales 1972;48(1):185-186.
12. Eldin M. Le climat en Cote d’Ivoire. Orstom 1971;107(2):78-108.
13. Thrusfield M, Ortega C, de Blas I, Noordhuizen JP, Frankena K. WIN EPISCOPE 2.0: improved epidemiological software for veterinary medicine. Vet Rec 2001;148(18):567-572 https://doi.org/10.1136/vr.148.18.567
14. Radostits O, Gay C, Hinchcliff K, Constable P. Veterinary Medicine: a Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. Saunders-Elsevier. Edinburgh, Scotland. 2007.
15. Njiru ZK, Constantine CC, Guya S, Crowther J, Kiragu JM, et al. The use of ITS1 rDNA PCR in detecting pathogenic African trypanosomes. Parasitol Res 2005;95(3):186-192 https://doi.org/10.1007/s00436-004-1267-5
16. Cox A, Tilley A, Mcodimba F, Fyfe J, Eisler M, et al. A PCR based assay for detection and differentiation of African trypanosome species in blood. Exp Parasitol 2005;111(1):24-29 https://doi.org/10.1016/j.exppara.2005.03.014
17. Ouédraogo-Sanon G. Thèse MmeOUEDRAOGO SANON Gisèle Version final; 27. 08. 2020. Universite Joseph Ki-Zerbo; 2020 (in French). Available from: https://www.researchgate.net/publication/344434317
18. Acapovi-Yao G, Mavoungou J, Kohagne L, Ta DTB. Dynamique et dispersion spatiale des Tabanidae dans différents faciès écologiques de Korhogo en Côte d’Ivoire. Entomol Faun-Faun Entomol 2017;70:13-22 (in French). https://doi.org/10.25518/2030-6318.3584
19. Desquesnes M. OIE Reference Laboratory for Animal Trypanosomoses of African origin Compendium of Standard Diagnostic Protocols for Animal Trypanosomoses of African Origin; 2017. 1-106 Available from: http://www.oie.int/nttat/Attachedfiles/A16-REC-COMPENDIUM_PROTOCOLES_TRYPANO-En.pdf
20. Djohan V, Kaba D, Rayaissé JB, Salou E, Coulibaly B, et al. Diversité spatio-temporelle des glossines le long du fleuve Comoé en Côte d’Ivoire. Rev d’élevage médecine vétérinaire des pays Trop 2015;68(1):39.
21. Djohan V, Kaba D, Rayaissé JB, Dayo GK, Coulibaly B, et al. Detection and identification of pathogenic trypanosome species in tsetse flies along the Comoé River in Côte d’Ivoire. Parasite 2015;22:18 https://doi.org/10.1051/parasite/2015018
22. Isaac C, Ciosi M, Hamilton A, Scullion KM, Dede P, et al. Molecular identification of different trypanosome species and subspecies in tsetse flies of northern Nigeria. Parasit Vectors 2016;9(1):1-7 https://doi.org/10.1186/s13071-016-1585-3
23. Tekle T, Terefe G, Cherenet T, Ashenafi H, Akoda KG, et al. Aberrant use and poor quality of trypanocides: A risk for drug resistance in south western Ethiopia. BMC Vet Res 2018;14(1):4 https://doi.org/10.1186/s12917-017-1327-6
24. Yapo EM. Analyse économique de la filière du médicament vétérinaire en Côte d’Ivoire; Universite Cheikh Anta Diop De Dakar; 2011. 130:(in French). Available from: https://beep.ird.fr/collect/eismv/index/assoc/TD11-17.dir/TD11-17.pdf
25. Boka OM, Boka EEJ, Yapi GY, Traoré SI, Kouamé KE. Epidémiologie de la trypanosomose animale africaine chez les bovins dans le département du Korhogo (Côte d’Ivoire). Rev d’élevage médecine vétérinaire des pays Trop 2019;72(2):83 (in French). https://doi.org/10.19182/remvt.31748
26. Yapi-Gnaore , Oya BA, Zana O. Revue de la situation des races d’ animaux domestiques De Cote d’ Ivoire. Anim Genet Resour génétiques Anim genéticos Anim 1996;19:91-108 (in French). https://doi.org/10.1017/S101423390000081X
27. Sokouri DP, Loukou NE, Yapi-Gnaoré CV, Mondeil F, Gnangbé F. Caractérisation phénotypique des bovins à viande (Bos taurus et Bos indicus) au centre (Bouaké) et au nord (Korhogo) de la Côte d’Ivoire. Anim Genet Resour Inf 2007;40:43-53 (in French). https://doi.org/10.1017/S1014233900002182
28. Goran EKN, Sokouri DP, Gnaore VCY, Fantodji AT. Croisement de la race n’ dama avec les races abondance et montbéliarde en zone tropicale humide de côte divoire: caractérisation phénotypique et analyse comparative des croisés pour leurs performances laitières en ferme. Agronomie Africaine 2015;27(1):15-26 (in French).
29. Dwinger RH, Clifford DJ, Agyemang K, Gettinby G, Grieve AS, et al. Comparative studies on N’Dama and zebu cattle following repeated infections with Trypanosoma congolense. Res Vet Sci 1992;52(3):292-298 https://doi.org/10.1016/0034-5288(92)90027-y
30. Dayo G, Bengaly Z, Messad S, Bucheton B, Sidibe I, et al. Prevalence and incidence of bovine trypanosomosis in an agro-pastoral area of southwestern Burkina Faso. Res Vet Sci 2010;88:470-477 https://doi.org/10.1016/j.rvsc.2009.10.010
Fig. 1Agarose gel electrophoresis of DNA samples amplified by ITS1 PCR. 1: Trypanosoma vivax (250 bp), 2: Trypanosoma simiae tsavo (400 bp). 3: Negative sample; 5 and 6: Trypanosoma congolense (700 bp). NC, Negative control; M, Marker. 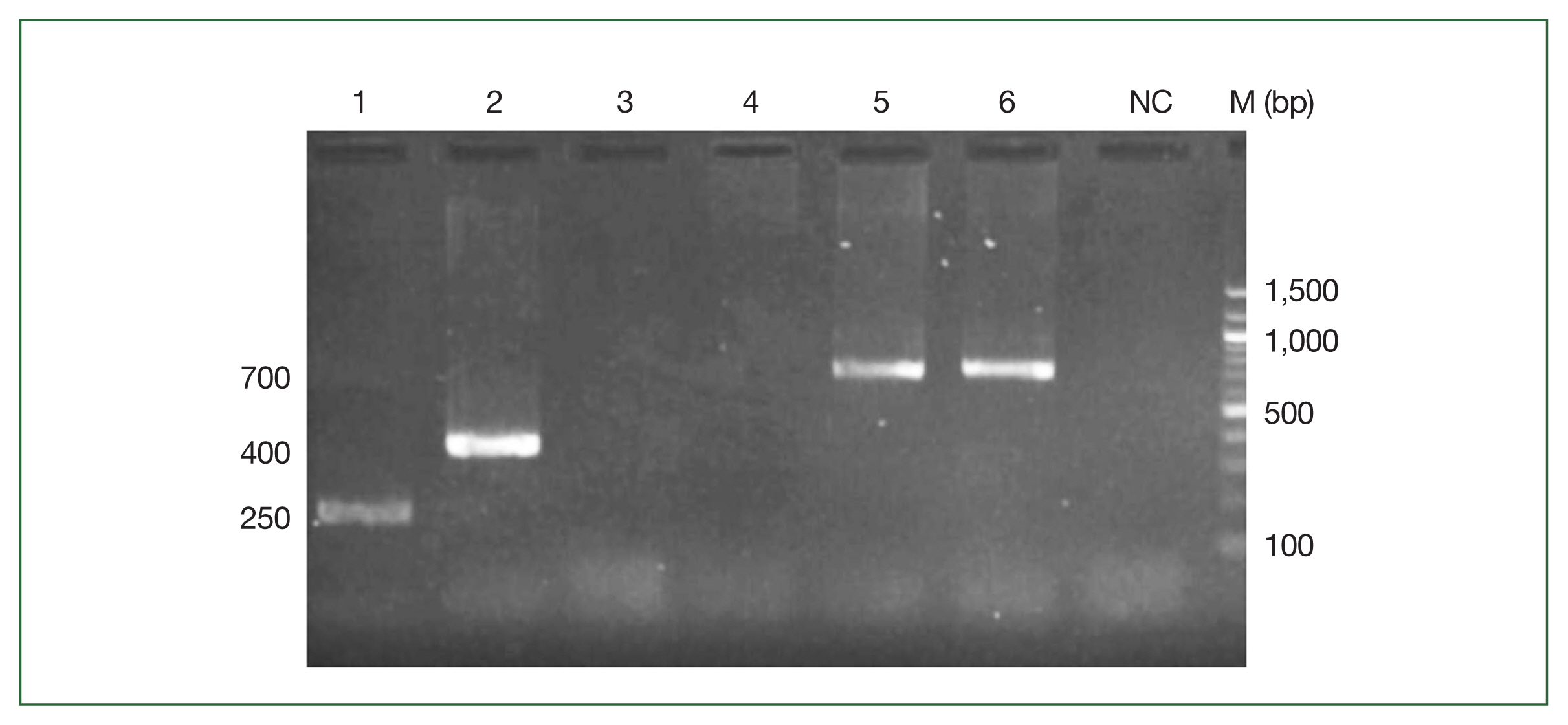
Table 1Prevalence according to diagnostics methods of trypanosome infections in the study population of cattle by region, department, race, age, and sex Table 2Effect of risk factors on animal infection |
|
|||||||||||||||||||||||||||||||||||||||||||||