Abstract
Toxoplasma gondii infections are primarily diagnosed by serological assays, whereas molecular and fluorescence-based techniques are garnering attention for their high sensitivity in detecting these infections. Nevertheless, each detection method has its limitations. The toxoplasmosis detection capabilities of most of the currently available methods have not been evaluated under identical experimental conditions. This study aimed to assess the diagnostic potential of enzyme-linked immunosorbent assay (ELISA), real-time polymerase chain reaction (RT-PCR), immunohistochemistry (IHC), and immunofluorescence (IF) in BALB/c mice experimentally infected with various doses of T. gondii ME49. The detection of toxoplasmosis from sera and brain tissues was markedly enhanced in mice subjected to high infection doses (200 and 300 cysts) compared to those subjected to lower doses (10 and 50 cysts) for all the detection methods. Additionally, increased B1 gene expression levels and cyst sizes were observed in the brain tissues of the mice. Importantly, IHC, IF, and ELISA, but not RT-PCR, successfully detected T. gondii infections at the lowest infection dose (10 cysts) in the brain. These findings may prove beneficial while designing experimental methodologies for detecting T. gondii infections in mice.
Introduction
Toxoplasma gondii infection in the host organism results in a highly prevalent parasitic disease called toxoplasmosis, which affects approximately a third of the global population [1]. The severity and clinical symptoms of T. gondii infection vary depending on the host’s immune response and the extent of the infection, respectively. T. gondii infections in immunocompetent individuals are frequently asymptomatic and characterized by mild and self-limiting general malaise. However, in pregnant women and immunocompromised individuals, the infection can result in neurological and ocular abnormalities in the fetus, encephalitis, and even death [1]. T. gondii has also been linked to various neurological and psychiatric disorders, such as schizophrenia and bipolar disorder, although evidence for its association with these diseases remains inconclusive [2]. Efficient and accurate detection of T. gondii infection is a crucial prerequisite for the development of management measures.
Infections caused by the T. gondii ME49 strain are primarily diagnosed by serological assays, particularly via ELISA, which determines the specific antibodies produced by the host’s immune system in response to the infection [3]. However, the detection of toxoplasmosis is not strictly limited to conventional ELISA-based methods owing to various advances in the field, which have enabled the application of a wide array of diagnostic methods. The hematoxylin and eosin staining method has been employed to delineate the morphology of cysts in murine brains infected by 20 cysts of T. gondii ME49 [4]. Similarly, immunohistochemistry (IHC) and immunofluorescence assay (IF) have been applied to detect T. gondii ME49 infection [5]. Moreover, the polymerase chain reaction (PCR) has been continually optimized as a promising detection method for these infections [6,7]. The use of the B1 gene and the ITS-1 region as primers in PCR was reported to be highly sensitive in quantifying T. gondii ME49 infections [8].
Numerous studies comparing the sensitivities of various toxoplasmosis detection assays have been reported [9–12], with each study being subjected to different experimental conditions. To the best of our knowledge, none of them has attempted to simultaneously determine T. gondii ME49 infections via ELISA, IHC, IF, and PCR under the same experimental conditions. Given the variable sensitivities of these techniques in detecting T. gondii ME49 infections in different laboratories and the conflicting findings reported in these literature [9–12], we reasoned that subjecting the diagnostic tests to identical experimental conditions would enable an accurate comparison of the detection methods. Furthermore, the relationships between the infection doses and the various identification approaches remain unclear.
This study aimed to detect T. gondii infections induced by different doses and observe the responses at various time intervals in the brains and/or sera of mice via ELISA, real-time PCR (RT-PCR), and cyst observations (IHC, IF). The techniques were assessed under identical conditions, thereby providing a reference for laboratory detection methods in future animal studies related to T. gondii ME49 infection.
Materials and MethodsEthics statementAll animal experiments were approved and performed by strictly adhering to the guidelines established by the Kyung Hee University IACUC (permission number: KHUASP(SE)-18-050).
Mice and parasitesSeven-week-old female BALB/c mice were obtained from NARA Biotech (Seoul, Korea) and maintained in a biosafety level 2 facility under appropriate temperature (25±2°C) and 12-h light-dark cycle settings. The T. gondii type II strain ME49 used in this study was maintained through serial passaging in BALB/c mice. Specifically, uninfected mice were inoculated with brain homogenates from previously infected mice, thereby preserving the parasite strain and ensuring a consistent source of infection. The infected mice were sacrificed to obtain cysts of T. gondii ME49 from the brain tissues before the new passage [13].
Challenge infection and sample collectionA total of 45 mice were randomly assigned to four groups (n=9 each) and orally infected with 100 μl of phosphate-buffered saline (PBS; 137 mM NaCl, 10 mM Na2HPO4, 2.7 mM KCl, and 1.8 mM KH2PO4) containing 10, 50, 200, or 300 T. gondii ME49 cysts. The mice were monitored weekly for changes in the body weight and survival rate for 84 days (equivalent to 3 months). Blood samples were collected from each mouse via retro-orbital plexus puncture 28 (month 1), 56 (month 2), and 84 (month 3) days post-infection (dpi). Three mice from each group were sacrificed at these time points, and the brain tissues were collected and stored at −80°C for further analysis.
Evaluation of the T. gondii ME49-specific IgG response via ELISABlood samples collected at regular intervals were centrifuged at 6,000 rpm for 10 min at 4°C for serum acquisition. Brain tissue supernatants were obtained by homogenizing the brain tissue in 3 ml syringes containing 200 μl of PBS and centrifuging them at 3,000 rpm for 20 min at 4°C, as described previously [14]. Sonicated ME49 lysate antigens were diluted in carbonate coating buffer (15 mM Na2CO3 and 34.8 mM NaHCO3) to a concentration of 4 μg/ml and incubated in a 96-well immunoplate overnight at 4°C to detect the T. gondii ME49-specific antibodies. The sera (1:100 dilution) and brain samples (1:10 dilution) were diluted in PBS containing 0.05% Tween 20 (PBST) and inoculated into each well at 37°C for 1 h. Horseradish peroxidase (HRP)-conjugated anti-mouse IgG secondary antibody (SouthernBiotech, Birmingham, AL, USA) was diluted in PBST (ratio, 1:2,000) and incubated at 37°C for 1 h. Color development was achieved by adding O-phenylenediamine substrate dissolved in citrate-phosphate buffer (pH 5.0; 0.1 M Na3C6H5O7 and 0.2 M Na2HPO4) containing 0.05% H2O2, and the reaction was stopped with H2SO4 [13]. Optical density readings were measured at 450 nm using a microplate reader (EZ Read 400, Biochrom, Cambridge, UK).
RT-PCR to determine the T. gondii B1 gene
T. gondii genomic DNA was extracted from the mouse brain samples using the genomic DNA Extraction Kit (Bioneer, Daejeon, Republic of Korea), according to the manufacturer’s instructions. The B1 gene’s repetitive single-copy sequence of the T. gondii ME49 strain was selected as the target DNA for amplification. The forward (5′-GGAGGACTGGCAACCTGGTGTCG-3′) and reverse (5′-TTGTTTCACCCGGACCGTTTAGCAG-3′) primers were designed using Oligo 6.0 software (Molecular Biology Insights, Colorado Springs, CO, USA) and synthesized by GenScript (Piscataway, NJ, USA). The GAPDH gene (forward primer: 5′-GGTGAAGGTCGGTGTGAACG-3′, reverse primer: 5′-CTCGCTCCTGGAAGATGGTG-3′) was used as a reference to normalize the expression levels of the target genes. In brief, a 20 μl reaction mix was prepared, comprising 1 μl of genomic DNA, 0.8 μl of 10 μM forward primer, 0.8 μl of 10 μM reverse primer, 0.8 μl of 10 μM probe, 0.4 μl of the Luna Universal Probe qPCR Master Mix (New England Biolabs, Rowley, MA, USA), and 7 μl of nuclease-free water. The micPCR Thermal Cycler (PhileKorea, Seoul, Korea) was used to perform the PCR procedure with the following reaction conditions: initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The relative expression level of the B1 gene was calculated using the 2−ΔΔCT method.
Isolation of T. gondii cysts
T. gondii ME49 cysts were obtained from the brain homogenates using a previously established method involving the Percoll density gradient [14]. Briefly, brain homogenates were subjected to centrifugation at 3,000 rpm for 20 min at 4°C. The pellets were resuspended in 44% Percoll and layered on top of the 67% Percoll solution. The mixture was centrifuged at 12,100 rpm for 20 min, and the layer at the interface of the different densities containing the T. gondii cysts was collected and washed with PBS. The purified cysts were then mounted on glass slides and quantified under a Leica DMi8 microscope (Leica Microsystems, Wetzlar, Germany).
IHC and IF staining for T. gondii detectionThe mice brains were fixed with 10% formaldehyde, embedded in paraffin wax, and cut into 4 μm-thick sections using a microtome. The sections were deparaffinized using xylene and rehydrated through a graded series of alcohol. Antigen retrieval was performed in 0.01 M citrate buffer (pH 6.0) for 10 min. Endogenous peroxidase was removed by incubating the sections in 3% H2O2 diluted in methanol for 10 min. After blocking with 5% BSA for 30 min, the sections were incubated (overnight at 4°C) with positive sera (1:500 dilution) collected from mice infected multiple times with T. gondii. After washing with TBST, the sections were incubated with HPR-conjugated IgG (1:2,000 dilution) for 30 min at room temperature, washed, and stained with 3,3′-diaminobenzidine (DAB, Sigma-Aldrich, St. Louis, MO, USA) for 5 min. Finally, the sections were counterstained with hematoxylin, dehydrated, and mounted with coverslips. IHC staining was visualized via light microscopy. For the IF staining, fluorescein-isothiocyanate (FITC)-conjugated IgG antibody (Abcam, Cambridge, UK; 1:300 dilution) was used as the secondary antibody. The intensity, location, and pattern of fluorescence were observed with a Leica DMi8 microscope.
Statistical analysisGraphPad Software version 9 (GraphPad Software, San Diego, CA, USA) was used for data analysis. Data are expressed as the mean±SD. Statistical distinctions between groups were assessed using one-way analysis of variance and Tukey’s post hoc test. P values less than 0.05 were considered statistically significant.
ResultsT. gondii ME49-specific IgG responses in the sera and brains of mice infected with varying doses of cystsThe antibody responses in the sera and brain supernatants of mice infected with varying doses of T. gondii ME49 cysts were determined at 28, 56, and 84 dpi using ELISA. As shown in Fig. 1A, the changes to the parasite-specific IgG after infection with 10 cysts were negligible during the first 2 months, but a significant increase compared to that in the naïve controls became apparent after 3 months. The antibody responses induced in mice were proportional to the infection dose, irrespective of the infection duration. The IgG antibody levels were significantly higher than those in the naïve controls as the infection dose was increased to 50, 200, and 300 cysts. Furthermore, administering high doses of T. gondii ME49 in the mice resulted in a markedly enhanced induction of IgG when compared to those in the naïve and the low infection dose groups. Consistent with the serum IgG data, the brain IgG responses were elicited in a dose-dependent manner, which progressively increased over the course of the infection (Fig. 1B). Unlike the serum antibody responses, the T. gondii-specific IgG responses in the brains of the infected mice were significantly higher than those in the uninfected controls at all time points. Furthermore, the brain IgG responses detected three months post-infection were significantly higher than those observed in the first and second months.
B1 gene levels in the brains of mice infected with varying doses of T. gondii ME49 cystsBrain tissues collected at 28, 56, and 84 dpi were utilized to examine the relative expression levels of the T. gondii B1 gene via RT-PCR (Fig. 2). The B1 gene expression levels in mice infected with either 10 or 50 T. gondii ME49 cysts were negligible compared to those in the naïve controls at the 3 time points. Conversely, significantly enhanced B1 gene expression was observed at all time points in mice infected with high doses of T. gondii cysts. Although a dose-dependent increase in B1 gene expression was detected, progressive increases over time were only seen in mice infected with 200 or 300 T. gondii cysts. Mice infected with higher doses (from 56 dpi with T. gondii infection and onward) exhibited B1 gene expression levels that were markedly greater than those in the mice infected with relatively lower T. gondii doses.
Parasite size, body weight changes, and survival of mice following T. gondii ME49 infectionsBrain tissue samples were collected from mice at 28, 56, and 84 dpi, and the cyst sizes were measured using a microscope (Fig. 3A). The cysts gradually enlarged over the course of infection, with the largest cyst size detected three months post-infection. Correlations between the cyst size and infection dose could only be drawn from 2 months post-infection onward because the size differences were negligible, regardless of the infection dose, during the first month of infection. Significant size differences were detected after 56 dpi; mice infected with 200 or 300 T. gondii ME49 generally possessed larger cysts than those inoculated with 10 or 50 cysts. Increasing the infection dose did not negatively influence the body weights of the mice (Fig. 3B). Consistent with the weight loss data, the T. gondii infections with varying doses of the cysts did not affect the survival of the mice during the 3-month infection period (Fig. 3C).
Staining image analysis in the brains of mice infected with different burdens of T. gondii ME49 cystsParaffin sections of the brain tissues were incubated with polyclonal mouse antibodies against T. gondii ME49. The corresponding secondary antibodies conjugated with HRP or fluorophore were used for IHC and IF, respectively. DAB-stained T. gondii cysts were not detected in the naïve mice (Fig. 4A). The cysts were too small to be detected during the first 2 months, irrespective of the infection dose (data not shown). However, at 84 dpi, they were detected and visualized as brown precipitates (Fig. 4B–E). The DAB-based IHC method successfully detected the T. gondii ME49 cysts, even at a dose of 10 cysts (Fig. 4B). Increasing the infection dose was associated with greater quantities and sizes of the cysts. Identical findings were observed from the IF images. Although FITC-stained cysts were undetected in the naïve group (Fig. 5A), they were detected in all the infection groups at 84 dpi (Fig. 5B–E). As with the IHC results, the cyst infection dose was proportional to the burden and size of the cyst.
DiscussionToxoplasmosis is a serious public health hazard that can have severe consequences depending on the immune status of the affected individual. Nonetheless, an effective commercially available vaccine for the prevention of human toxoplasmosis remains unavailable [15]. Therefore, conducting preclinical investigations of T. gondii using animals is a necessary precondition for developing effective regulation strategies; moreover, the research quality is heavily dependent on the sensitivity of the detection techniques. Our study investigated the relationship between different infection doses and the corresponding responses at various time points after infections, which is the first study to detect T. gondii infection using 4 techniques (ELISA, RT-PCR, IHC, and IF) under the same experimental conditions. Understanding the sensitivity and applicability of these methods at varying infection doses and time courses may provide a valuable reference for selecting optimal study strategies.
Although several previous studies have reviewed data acquired from a substantial num ber of patients infected with distinct T. gondii genotypes, a direct correlation between the parasite burden and the severity of the related disease could not be drawn [16–18]. However, animal experiments exhibit distinguishable characteristics. In the present study, changes in the infection dose were reflected in several test results, with unsatisfactory results in the low-dose infection groups. Specifically, the serum IgG levels in mice infected with a high dose of the parasite (200 and 300 cysts) were consistently higher than those in mice infected with a low dose (10 and 50 cysts), irrespective of the infection duration. This was consistent with the findings previously reported using other ELISA-based methods, such as the chemiluminescence assay (CLIA), enzyme-linked fluorescence assay, immunochromatographic test (ICT), serum IgG avidity test, and immunosorbent agglutination assay, all of which exhibited high sensitivity [19]. The efficacy can be further enhanced by utilizing recombinant or chimeric antigens and multiepitope peptides with promising outcomes [20]. From the second month onward, we observed a similar trend between the levels of the B1 gene and the cyst size in the high-dose infected mice, which was not evident during the early stage of infection (first month). These findings suggest that, during the early stage of infection, particularly within the first month, ELISA may be more sensitive in identifying and differentiating the high and low infectious doses compared to PCR and visual observations in the brain tissues of mice. Additionally, the effectiveness of all these methods is influenced by the infection dose.
The ME49 strain is commonly used for T. gondii research in animal models. Once it infects the intestinal epithelial cells, the parasites disseminate throughout the body and reach the brain, forming tissue cysts that persist quiescently for life [21]. A previous study have shown that cysts begin to appear in the brains of mice infected with 20 ME49 cysts within one-week post-infection, and immunohistology methods have been reported to detect the cysts during the early stages of infection [22]. However, detection in cases with as few as 10 cysts was rarely reported. In the current study, the cysts could not be detected using IHC during the early stages of infection, probably due to the low infection dose, which elicited a less potent immune response, and the unfavorable environmental conditions within the gastrointestinal tract [23]. The only way to detect T. gondii infections with such low doses was through IgG tests in the brain; the cysts lasted up to 3 months after infection in the brain but were not detected in the sera of the mice. During T. gondii infection, parasite-specific antibodies are produced by antibody-secreting plasma cells in the secondary lymphoid organs and distributed throughout the body. In addition, IgG antibodies are directly produced by circulating B cells that cross the blood-brain barrier, after being induced by cytokine and chemokine signaling [24,25]. The findings of the present study suggest that the latter mechanism may be dominant in extremely low-dose infections. In cases with low-dose cysts, the intensity of the B cells recruited by the infected brain cells and the antibody immunity produced exceed that of antibodies present in the blood during the early stage; thus, the detection of IgG in the brain is more sensitive at low infection doses. This was indirectly proved by the presence of CD45R (B220)+ IgG-producing B cells in the brain tissues of mice as early as 1 month after infection [26]. Moreover, consistent with our report, T. gondii-specific IgG antibodies have been found in the cerebrospinal fluid of infected baboons 10 days after infection [27]. This evidence serves as a reference for the development of efficient detection techniques in forthcoming animal experiments involving low-dose T. gondii infections.
Although parasite cysts and DNA replication have been shown to increase in size over time during high-dose infections, the mice in the current study maintained their body weight and survived until 84 dpi. This may be because although the dose of 300 cysts was considered high in this study, it was relatively low compared to our previous studies where mice were challenged orally with 450 or 2,000 cysts, and all the infected mice died before 50 or 16 dpi, respectively [13,28]. Thus, the survival time may be inversely correlated to the infection dose, which must be considered when conducting related immunology or pharmacology experiments in the future.
In conclusion, the present study describes various identification techniques, including ELISA, B1 gene detection, and cyst quantity, to evaluate T. gondii ME49 infections in mice. Additionally, the efficacy was compared using varying cyst doses at distinct time points. The findings of this study may provide an experimental basis for the selection of detection methods for T. gondii infections in animals. The development of rapid test measures that do not compromise the accuracy of the assay while providing reproducible results within a short time is of great significance for the quality of basic studies. Hence, it is imperative to comprehensively evaluate the advantages and disadvantages of each detection method in order to determine the most suitable strategy under specific conditions.
AcknowledgmentsThis study was financially supported by the Core Research Institute (CRI) Program, the Basic Science Research Program through the National Research Foundation of Korea (NRF), Ministry of Education (NRF2018R1A6A1A03025124).
NotesAuthor contributions
Conceptualization: Kang HJ, Kim MJ, Quan FS
Formal analysis: Kang HJ, Mao J, Yoon KW
Funding acquisition: Moon EK
Methodology: Kang HJ, Mao J, Kim MJ, Yoon KW, Eom GD, Chu KB
Resources: Moon EK, Quan FS
Software: Mao J, Eom GD
Supervision: Quan FS
Visualization: Kim MJ
Writing – original draft: Mao J
Writing – review & editing: Chu KB, Quan FS
References1. Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke 2018;13(6):612-632
https//doi.org/10.1177/1747493018778713
2. Nayeri T, Sarvi S, Daryani A. Toxoplasmosis: targeting neurotransmitter systems in psychiatric disorders. Metab Brain Dis 2022;37(1):123-146
https//doi.org/10.1007/s11011-021-00824-2
3. Jones JL, Dargelas V, Roberts J, Press C, Remington JS, et al. Risk factors for Toxoplasma gondii infection in the United States. Clin Infect Dis 2009;49(6):878-884
https//doi.org/10.1086/605433
4. Suzuki Y, Wang X, Jortner BS, Payne L, Ni Y, et al. Removal of Toxoplasma gondii cysts from the brain by perforin-mediated activity of CD8+ T cells. Am J Pathol 2010;176(4):1607-1613
https//doi.org/10.2353/ajpath.2010.090825
5. Torres L, Robinson SA, Kim DG, Yan A, Cleland TA, et al.
Toxoplasma gondii alters NMDAR signaling and induces signs of Alzheimer’s disease in wild-type, C57BL/6 mice. J Neuroinflammation 2018;15(1):57
https//doi.org/10.1186/s12974-018-1086-8
6. Evangelista FF, Costa-Ferreira W, Mantelo FM, Beletini LF, de Souza AH, et al. Rosuvastatin revert memory impairment and anxiogenic-like effect in mice infected with the chronic ME-49 strain of Toxoplasma gondii
. PLoS One 2021;16(4):e0250079
https//doi.org/10.1371/journal.pone.0250079
7. Hegazy MK, Saleh NE, Aboukamar WA. Detection of chronic toxoplasmosis in the brain of mice using loop-mediated isothermal amplification (LAMP) and conventional PCR. Exp Parasitol 2023;251:108556
https//doi.org/10.1016/j.exppara.2023.108556
8. Rahumatullah A, Khoo BY, Noordin R. Triplex PCR using new primers for the detection of Toxoplasma gondii
. Exp Parasitol 2012;131(2):231-238
https//doi.org/10.1016/j.exppara.2012.04.009
9. Bezerra ECM, Dos Santos SV, Dos Santos TCC, de Andrade HFJ, Meireles LR. Behavioral evaluation of BALB/c (Mus musculus) mice infected with genetically distinct strains of Toxoplasma gondii.
. Microb Pathog 2019;126:279-286
https//doi.org/10.1016/j.micpath.2018.11.021
10. Kikuchi T, Furuta T, Kojima S. Kinetics of the nucleoside triphosphate hydrolase of Toxoplasma gondii in mice with acute and chronic toxoplasmosis. Ann Trop Med Parasitol 2002;96(1):35-41
https//doi.org/10.1179/000349802125000493
11. Rahumatullah A, Khoo B, Noordin R. Development of triplex real-time PCR and detection of Toxoplasma gondii DNA in infected mice tissues and spiked human samples. Trop biomed 2015;32(2):376-385.
12. Piña-Vázquez C, Saavedra R, Herion P. A quantitative competitive PCR method to determine the parasite load in the brain of Toxoplasma gondii-infected mice. Parasitol Int 2008;57(3):347-353
https//doi.org/10.1016/j.parint.2008.03.001
13. Yoon KW, Chu KB, Kang HJ, Kim MJ, Eom GD, et al. Mucosal administration of recombinant baculovirus displaying Toxoplasma gondii ROP4 confers protection against T. gondii challenge infection in mice. Front Cell Infect Microbiol 2021;11:735191
https//doi.org/10.3389/fcimb.2021.735191
14. Kang HJ, Lee SH, Kim MJ, Chu KB, Lee DH, et al. Influenza virus-like particles presenting both Toxoplasma gondii ROP4 and ROP13 enhance protection against T. gondii infection. Pharmaceutics 2019;11(7):342
https//doi.org/10.3390/pharmaceutics11070342
15. Chu KB, Quan FS. Advances in Toxoplasma gondii vaccines: current strategies and challenges for vaccine development. Vaccines (Basel) 2021;9(5):413
https//doi.org/10.3390/vaccines9050413
16. Dubey JP. Outbreaks of clinical toxoplasmosis in humans: five decades of personal experience, perspectives and lessons learned. Parasit Vectors 2021;14(1):263
https//doi.org/10.1186/s13071-021-04769-4
17. Karshima SN, Karshima MN. Human Toxoplasma gondii infection in Nigeria: a systematic review and meta-analysis of data published between 1960 and 2019. BMC Public Health 2020;20(1):877
https//doi.org/10.1186/s12889-020-09015-7
18. Strang A, Ferrari RG, do Rosário DK, Nishi L, Evangelista FF, et al. The congenital toxoplasmosis burden in Brazil: systematic review and meta-analysis. Acta Trop 2020;211:105608
https//doi.org/10.1016/j.actatropica.2020.105608
19. Rostami A, Karanis P, Fallahi S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection 2018;46(3):303-315
https//doi.org/10.1007/s15010-017-1111-3
20. Ferra BT, Holec-Gąsior L, Gatkowska J, Dziadek B, Dzitko K, et al. The first study on the usefulness of recombinant tetravalent chimeric proteins containing fragments of SAG2, GRA1, ROP1 and AMA1 antigens in the detection of specific anti-Toxoplasma gondii antibodies in mouse and human sera. PLoS One 2019;14(6):e0217866
https//doi.org/10.1371/journal.pone.0217866
21. Attias M, Teixeira DE, Benchimol M, Vommaro RC, Crepaldi PH, et al. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit Vectors 2020;13(1):588
https//doi.org/10.1186/s13071-020-04445-z
22. Watson GF, Davis PH. Systematic review and meta-analysis of variation in Toxoplasma gondii cyst burden in the murine model. Exp Parasitol 2019;196:55-62
https//doi.org/10.1016/j.exppara.2018.12.003
23. Di Cristina M, Marocco D, Galizi R, Proietti C, Spaccapelo R, et al. Temporal and spatial distribution of Toxoplasma gondii differentiation into bradyzoites and tissue cyst formation in vivo. Infect Immun 2008;76(8):3491-3501
https//doi.org/10.1128/iai.00254-08
24. Suzuki Y, Lutshumba J, Chen KC, Abdelaziz MH, Sa Q, et al. IFN-γ production by brain-resident cells activates cerebral mRNA expression of a wide spectrum of molecules critical for both innate and T cell-mediated protective immunity to control reactivation of chronic infection with Toxoplasma gondii.
. Front Cell Infect Microbiol 2023;13:1110508
https//doi.org/10.3389/fcimb.2023.1110508
25. Sasai M, Yamamoto M. Innate, adaptive, and cell-autonomous immunity against Toxoplasma gondii infection. Exp Mol Med 2019;51(12):1-10
https//doi.org/10.1038/s12276-019-0353-9
26. Schlüter D, Kaefer N, Hof H, Wiestler OD, Deckert-Schlüter M. Expression pattern and cellular origin of cytokines in the normal and Toxoplasma gondii-infected murine brain. Am J Pathol 1997;150(3):1021-1035.
27. Araujo FG, Chiari E, Dias JC. Demonstration of Trypanosoma cruzi antigen in serum from patients with Chagas’ disease. Lancet 1981;1(8214):246-249
https//doi.org/10.1016/s0140-6736(81)92088-2
28. Kim MJ, Lee SH, Kang HJ, Chu KB, Park H, et al. Virus-like particle vaccine displaying Toxoplasma gondii apical membrane antigen 1 induces protection against T. gondii ME49 infection in mice. Microb Pathog 2020;142:104090
https//doi.org/10.1016/j.micpath.2020.104090
Fig. 1Antibody responses in sera and brains of BALB/c mice experimentally infected with T. gondii. (A) Sera and (B) brains were collected at 28, 56, and 84 (equivalent to months 1, 2, and 3) days in mice post-infections and parasite-specific IgG response was determined using ELISA. T. gondii lysate antigen was utilized as coating antigens. The data were presented as mean±SD, which were obtained from three independent experiments. *P<0.05, **P<0.01, and ***P<0.001 compared to the naïve control. 
Fig. 2
T. gondii B1 gene levels in brain tissues of mice exerimentally infected with T. gondii. Real-time PCR was performed to determine the relative expression of the T. gondii B1 gene in mice infected with varying doses of ME49 cysts. Brain samples were collected at months 1, 2, and 3 after infections for the analysis. The fold change in gene expression was calculated using the 2−ΔΔCT method with GAPDH as the reference gene. The results are presented as mean±SD and significance was indicated using *P<0.05, **P<0.01, and ***P<0.001. 
Fig. 3Cysts size of the parasite, and body weight changes and survival of mice after infections. The mice were infected with varying doses of T. gondii ME49 strain. The cysts were separated from the brain tissues of the mice at 28, 56, and 84 days post-infections and their size was measured (A). The changes in body weight (B) and survival (C) of mice were recorded weekly until day 84. Representative data from three independent experiments were presented as mean±SD, with *P<0.05 and **P<0.01 denoting statistical significance compared to the naïve control. 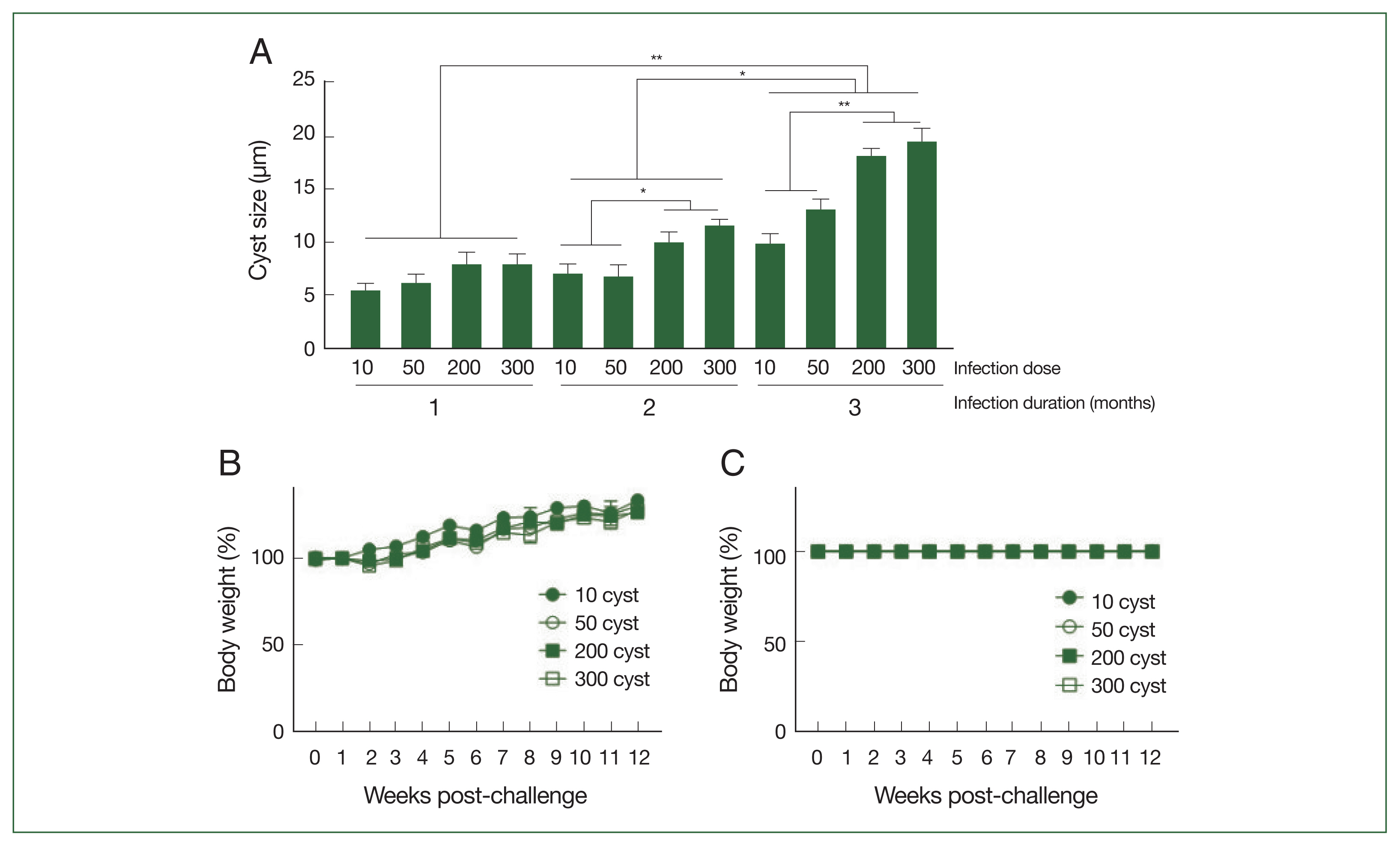
Fig. 4Parasite loads in mice brains are observed by immunohistochemical staining. Brain sections were obtained on 84 days after infections with 0 (A, naïve control), 10 (B), 50 (C), 200 (D), or 300 (E) T. gondii ME49 cysts. The cysts were probed with anti-T. gondii polyclonal antibody. We used 3,3′-diaminobenzidine (DAB) chromogen. Representative image from each group was provided. All images were acquired at 200×magnification (bar=20 μm). 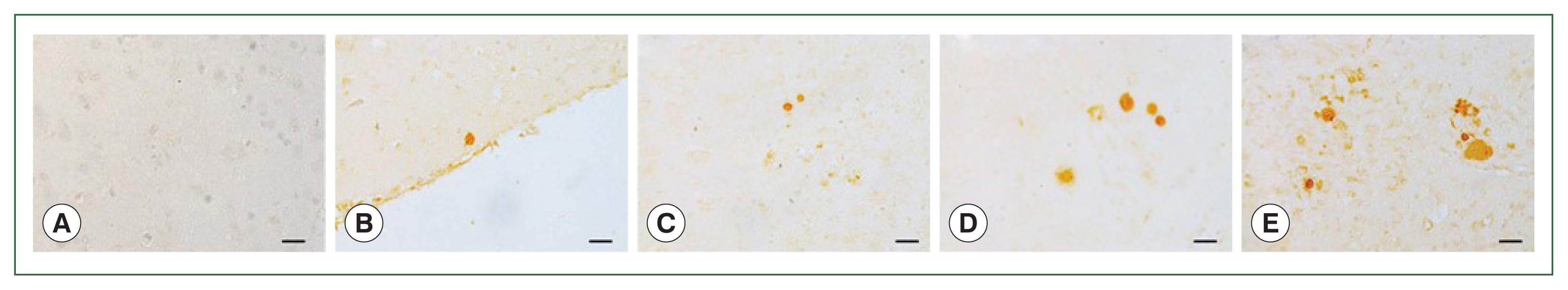
Fig. 5
T. gondii ME49 cysts loaded in mice brains are evaluated by immunofluorescence (IF) analysis. IF assays were used to assess the parasite loads in the brains of T. gondii-infected mice with 0 (A), 10 (B), 50 (C), 200 (D), and 300 (E) cysts at 84 dpi. Anti-T. gondii polyclonal antibody was used as the primary antibody, and fluorescein-isothiocyanate (FITC)-IgG was used as the secondary antibody. Brain sections obtained from 3 independent experiments were visualized using a fluorescent microscope (bar=20 μm). Representative image from each group was provided. All images were acquired at 200×magnification. 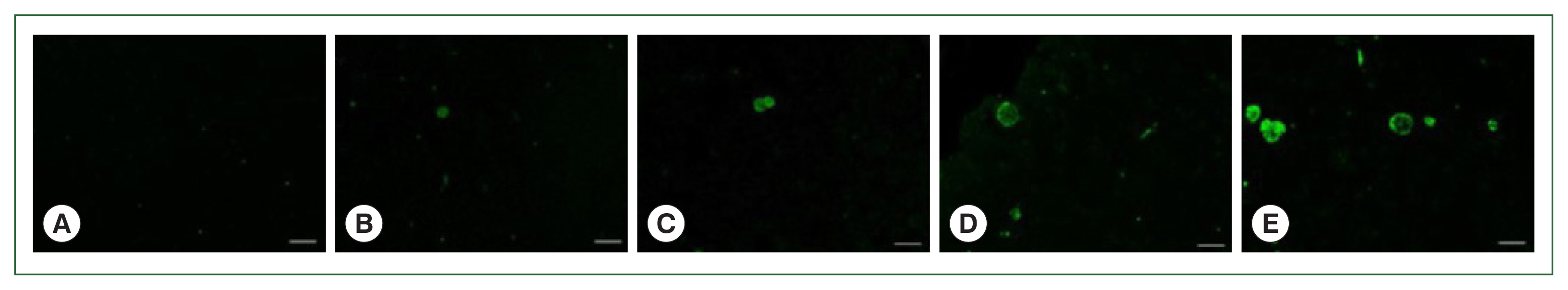
|
|
|||||||||||||||||||||||||||||||||||||